Unveiling the Chemistry Behind Lead-Acid Batteries
Have you ever stopped to ponder the intricate processes unfolding within the robust confines of lead-acid batteries? Within these unassuming casings lies a bustling chemical dance, orchestrating the energy we rely on. Let's delve into the enigma surrounding the secret life inside lead-acid batteries through a concise exploration.
How Lead Plates Generate Electricity in Sulfuric Acid:
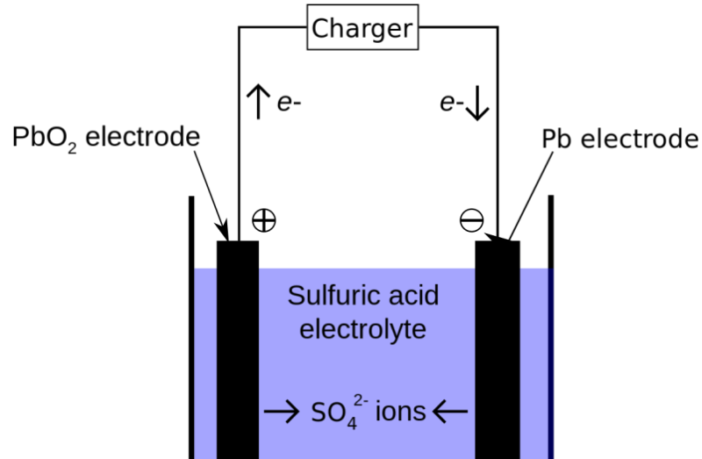
Deep within lead-acid batteries reside an array of cells, each housing a pair of plates - positive and negative electrodes, with diluted sulfuric acid serving as their divider. When fully charged, each cell delivers approximately 2.1 volts. For a 6-volt battery, three cells are necessary, and for a 12-volt battery, six cells suffice.
The lead plates within each cell are intricately crafted grids with marginally different chemical compositions, coated with distinct active materials. As the battery is utilized, it undergoes discharge, releasing stored energy and consuming portions of sulfuric acid. Gradually, sulfate from the acid blankets the plates, reducing the available surface for the chemical reaction to persist. Once fully coated, the reaction halts, rendering the battery powerless.
Despite reaching this point, there's hope for the resurrection of lead-acid batteries through recharging. Upon recharging, the sulfate returns to the acid, allowing the process to recommence. However, not all sulfate is fully reclaimed with each cycle, leaving a portion on the plates.
In essence, while lead-acid batteries offer indispensable energy storage, their lifespan is finite. Yet, understanding the intricate chemistry within these batteries sheds light on their operation and the possibility of extending their longevity through careful maintenance and recharging. Such is the fascinating journey within the heart of lead-acid batteries - a dance of chemicals shaping our energy needs.

